Introducing Our New Membrane-Based Water for Injection (WFI) Skids
- Share on Facebook
- Share on LinkedIn
- Share on Email
-
Copy Link
-
Share Link
- Pharmaceutical
- January 2, 2025
- 2 Minute Read
- Share on Facebook
- Share on LinkedIn
- Share on Email
-
Copy Link
-
Share Link
With our unwavering dedication to quality and the use of off-the-shelf, non-proprietary components for simplified maintenance, these thoroughly tested and seamlessly integrated skids offer effortless installation, operation, and servicing.
Traditionally, we've produced Water for Injection (WFI) for the pharmaceutical industry by distilling feedwater with plant steam and condensing it with cooling water—a reliable method still offered through our Multiple Effect and Single Effect Stills. However, updates to the European Pharmacopoeia now allow equivalent alternatives.
To give our customers more options, we're launching membrane-based WFI skids. Our solution features modular skids with a unified control screen, transforming your potable water into WFI.
We integrate softeners, carbon filters, reverse osmosis, deionization, ultrafiltration, and UV purification into a space-efficient package. Designed for pharmaceutical needs, our system includes hot water-sanitizable RO membranes, in-skid water recycling, UV-sanitized carbon filters, fusion-welded polypropylene tubing for the softener skid, and polished stainless steel tubing for the RO and UF skids.
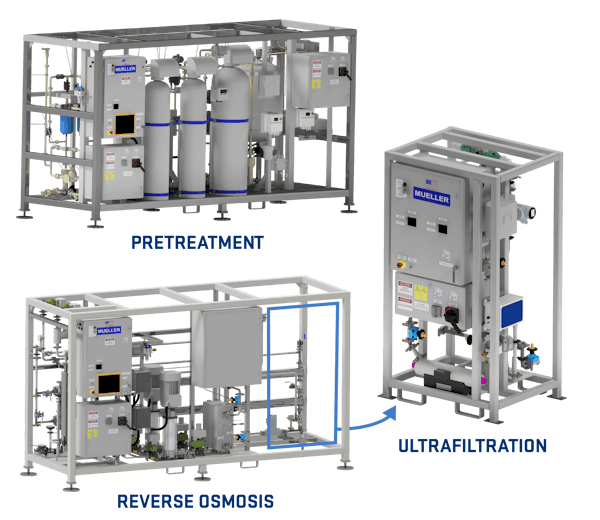
Our membrane-based Water for Injection (WFI) skids operate at ambient temperature, allowing you to align production and distribution temperatures seamlessly. This eliminates the need for extra heating or cooling of loop water, reducing utility costs and delivering a more sustainable solution. Plus, unlike traditional methods, our system requires no plant steam or coolant for WFI production—simplifying your process and cutting operational expenses.
Each skid comes complete with our comprehensive pharmaceutical documentation package, ensuring full compliance and traceability for your peace of mind.
Skids are provided with our full pharmaceutical documentation package, including:
- P&ID (Piping and Instrumentation Diagram)
- General arrangement drawing
- Electrical schematics
- Material test reports and surface finish certs for sanitary components
- USP Class VI elastomers for sanitary components
- Calibration certificates
- Sanitary weld records
- Component manuals
- Cut sheets
- Full bill of materials
- Functional specifications
- Software design specification
- Factory acceptance testing (FAT)
- Site acceptance testing (SAT)
More from Mueller Academy
- Manway Gasket Installation & Bushing Adjustment
- How to Decide Between a Horizontal & Vertical Milk Tank
- MES & PSG Feedwater Quality Requirements
- On-Site Tank Fabrication Under Deadline
- Finding the Right Finish: Understanding Polishing
- Increase the Capacity of Your Clean Utility Equipment
- Balancing a Mueller Multiple Effect Still (MES)
- The Different Types of Steam in Industrial Steam Generation
- How a Pure Steam Generator Works